Glycosylation of immunoglobulin G is regulated by a large network of genes pleiotropic with inflammatory diseases
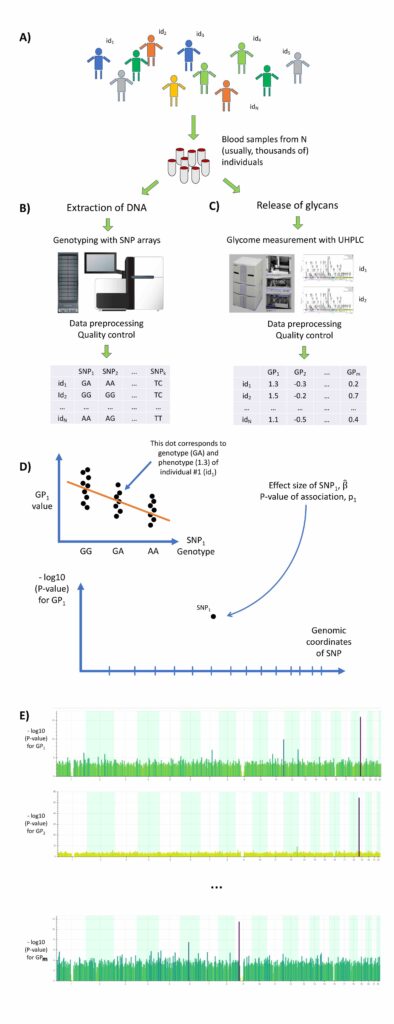
Immunoglobulin G (IgG) that plays central role in adaptive immunity is the most abundant immunoglobulin found in human plasma. All IgG molecules are N-glycosylated. N-glycosylation of IgG contributes to the maintenance of its structure, stability, and solubility. In a large collaborative study, we analysed association between genomes and glycosylation of immunoglobulin G (IgG) in more than 8,000 people from four European countries1. The study revealed 33 different regions of our genome that regulate composition of the IgG glycome. IgG glycosylation appears to be regulated by a complex network of genes that are also known risk factors for many diseases including inflammatory bowel disease, systemic lupus erythematosus, rheumatoid arthritis, primary biliary cirrhosis, asthma, and others.
It is common knowledge that genes influence our traits. Genes are DNA blueprints that are used to build proteins – molecules that are essential for life. Proteins perform many different functions in our body, such as important structural roles (for example our hair is built from proteins called keratins), defensive roles (e.g. antibodies that protect us from infections), roles in transport (haemoglobin that carries oxygen in our blood cells) and roles in chemical reactions (for example enzymes that help us digest our food). Glycans are sugars attached to the surface of a protein which can affect the function of the protein and how it binds to other molecules.
Immunoglobulins are specialised proteins that play the role of bodyguards in our immune system. When foreign substances (such as bacteria or viruses) enter our body, IgG signals to the immune system that it should act to remove these. Based on the type of glycan bound to IgG, this signal can act either to induce or suppress inflammation.
In this work, we have measured the profile of IgG N-glycosylation in more than 8,000 samples. We then studied association between abundances of different glycans, as characterised by 77 traits, and genetic polymorphisms using genome-wide association study approach (see Figure). Altogether, thirty-three different regions of DNA were highlighted as influencing the glycans on IgG. We demonstrated that some of the variants affecting IgG-glycosylation also likely modify the risk for inflammatory bowel disease, rheumatoid arthritis, primary biliary cirrhosis, and asthma.
Knowing which genes are involved in IgG glycosylation improves our understanding of how this process affects disease risk. It was already known that glycans are altered in various diseases, but it remains unclear exactly why this is. This study, published in Science Advances1, helps to shed light on the different molecules that are involved in these complex processes. This, in turn, can help develop new biomarkers of disease or suggest new drug targets.
(drafted in Feb. 2020, published in final form in Dec 2020 by YA)
- 1.Klarić L, Tsepilov YA, Stanton CM, et al. Glycosylation of immunoglobulin G is regulated by a large network of genes pleiotropic with inflammatory diseases. Sci Adv. Published online February 2020:eaax0301. doi:10.1126/sciadv.aax0301
No Comments